At Alexion, our mission is to transform the lives of people affected by rare diseases and devastating conditions. In addition to developing and delivering life-changing medicines, this mission drives us to seek opportunities to advance innovative solutions to system-wide challenges faced by rare disease patients, such as the “diagnostic odyssey.”
What is the Diagnostic Odyssey?
There are 400 million people around the world who are affected by a rare disease, half of whom are children. Often these patients wait years to receive an accurate diagnosis – and many never receive one at all. With three in 10 children who have a rare disease not living to see their 5th birthday, a fast and accurate diagnosis and pathway to care is of critical importance – every day can make a difference.
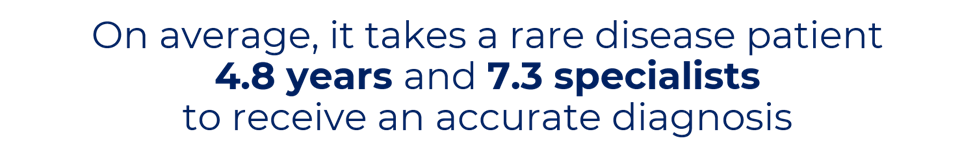
Today, we have more medical data than ever before, yet much of it is untapped, disorganized, and unusable. With more than 7,000 known rare diseases in the world, it can be difficult for physicians to correctly identify a diagnosis. Even when a correct diagnosis is made, physicians may not have access to the best available medical information that can inform a patient’s care plan.
Addressing the Problem with RARE ANSWERS™
RARE ANSWERS™ is a system of innovative and sustainable tools designed to help shorten the diagnostic journey and time to initiating treatment for children with a rare disease. By leveraging the best available medical information for rare diseases, clinical insights, and precision software, RARE ANSWERS provides physicians with a guided approach to patient assessment and diagnosis by mapping a patient’s phenotypic profile with genetic testing results to deliver an accurate diagnosis, quickly. These tools are designed to deliver actionable data to healthcare providers seeking the best care plan available for each patient. Because RARE ANSWERS is both personalized and scalable, it has the potential to be beneficial to patients and physicians regardless of geography and other factors.
Developed in collaboration with leading children’s hospitals and technology and data-science companies, pilots of this approach have achieved world records in reducing the time to diagnosis and treatment for children in NICUs. Alexion has acted as steward of this program for five years, investing as part of our commitment to meeting the needs of all rare disease patients.
A Closer Look at the Future of Genomic Medicine
Programs like RARE ANSWERS have the potential to impact rare disease care for thousands of children and their families. We encourage you to visit the Rady Children's Hospital Foundation website to read the story of one newborn baby boy who received a life-saving diagnosis as the result of rapid whole genome sequencing.
RARE ANSWERS Resources
For more information on RARE ANSWERS tools, download the fact sheet and FAQs, read the press releases announcing Alexion’s collaboration with Rady Children’s Institute for Genomic Medicine and The Manton Center at Boston Children’s Hospital, or visit the Research and Development page of Alexion.com.
Alexion has also contributed to Diagnosis of genetic diseases in seriously ill children by rapid whole-genome sequencing and automated phenotyping and interpretation.
• https://www.sciencedirect.com/science/article/pii/S2666247721000166?via%3Dihub
• https://www.nejm.org/doi/10.1056/NEJMc2100365
• https://stm.sciencemag.org/content/11/489/eaat6177.abstract.
Other publications that support RARE ANSWERS include:
- An RCT of Rapid Genomic Sequencing among Seriously Ill Infants Results in High Clinical Utility, Changes in Management, and Low Perceived Harm
The American Journal of Human Genetics 2020 https://dx.doi.org/10.1016/j.ajhg.2020.10.003
- Prospective, phenotype-driven selection of critically ill neonates for rapid exome sequencing is associated with high diagnostic yield
Genetics in Medicine 2020 https://dx.doi.org/10.1038/s41436-019-0708-6
- A longitudinal footprint of genetic epilepsies using automated electronic medical record interpretation
Genetics in Medicine 2020 https://www.nature.com/articles/s41436-020-0923-1
- Project baby bear pilot study implemented across the State of California to assess clinical impact of rapid precision medicine program https://radygenomics.org/wp-content/uploads/2020/07/PBB-Final-Report_07.14.20.pdf
- Detecting rare diseases in electronic health records using machine learning and knowledge engineering: Case study of acute hepatic porphyria
PLOS ONE 2020 https://dx.doi.org/10.1371/journal.pone.0235574
- Children’s rare disease cohorts: an integrative research and clinical genomics initiative
npj Genomic medicine 2020 https://www.nature.com/articles/s41525-020-0137-0